OnQ joins the P95 family

October 16, 2023. Leuven, Belgium and Johannesburg, South Africa - P95 BV (“P95”), a portfolio company of Ampersand Capital Partners and leading provider of clinical and observational services to vaccine developers, has signed an agreement to merge OnQ Research (“OnQ”) into P95. The transaction, which is subject to regulatory approval and scheduled to close before year end, furthers P95’s goal of becoming the leading global, vaccine-focused CRO. P95 now has unparalleled expertise conducting research in emerging geographies on the infectious diseases which could possibly drive a future pandemic. The combined company will also continue to offer clinical research services in disease areas beyond its vaccine focus (e.g. oncology, medical devices, cardiology).
Upon completion of the merger, the combined P95-OnQ will have a physical presence in over 30 countries with offices in Europe, Latin America, Asia, and Africa. Its services will cover both regulatory- grade real-world evidence and full-service interventional clinical trial research. Over the last 20+ years, the respective expertise of both organizations has been applied at the cutting edge of vaccine research for both bacterial and viral diseases including Influenza, Covid-19, Group B Strep, RSV, Hepatitis variants, and E Coli.
“This is an exciting transaction for our respective teams and clients, and an important step towards our goal of being the preferred service provider to all vaccine stakeholders,” said Thomas Verstraeten, CEO of P95. “The enhanced geographic footprint of the combined company will accelerate recruitment timelines, while providing our sponsor partners access to a global, diverse patient base. Further, the expansion into Africa for P95’s ongoing real world evidence projects provides a pathway to generate unique insights for both R&D prioritization and the monitoring of public health decision effectiveness.”
Catherine Lund, CEO of OnQ, said “We have grown exponentially in recent years and have proudly built a company whose quality standards are on par with those seen at North American and European CROs. Africa has enormous potential to contribute to global infectious disease research, and we believe merging with P95 will help accelerate the continent’s impact on the field. Together we will implement state-of-the-art clinical trial infrastructure and help establish the combined P95-OnQ as the unrivaled African champion in infectious disease clinical research services. Additionally, we see strong cultural alignment between the two companies to pursue sustainable economic empowerment which addresses the socioeconomic disparities present in the regions in which we operate.”
Benoit Bouche, Chairman of the Board at P95 and an Operating Partner at Ampersand Capital Partners, added, “I am proud to support a company with such high potential for positive contribution to the global struggle against infectious disease. We will pursue our mission with the ultimate goal of improving global access to effective vaccines.”
About P95
P95 is a global company headquartered in Leuven (Belgium) and with local and regional offices located in Rotterdam (the Netherlands), Bogota (Colombia) and Bangkok (Thailand). P95 delivers cutting-edge expertise in epidemiology and pharmacovigilance. The P95 team is comprised of epidemiologists, pharmacovigilance specialists, biostatisticians, data scientists, medical writers, IT engineers and study managers, located in over 17 countries. P95 projects are largely focused on a broad range of infectious diseases and vaccines, and the company also offers customized services upon request. Additional information about P95 is available at www.p-95.com.
Quantitative benefit/risk assessment of the HPV vaccine for preventing anal cancer in males
Quantitative benefit/risk assessment of the HPV vaccine for preventing anal cancer in males
Human Papillomaviruses (HPVs) are the primary pathogens causing anal cancers, accounting for nearly 90% of global cases. The HPV-associated anal cancers and deaths are worryingly increasing in men but can be prevented with the quadrivalent HPV (qHPV) vaccine. P95 estimated the benefit-risk balance of qHPV vaccine use in males, applying a multicriteria decision analysis (MCDA). The qHPV vaccine was shown to have a satisfactory BR balance in males, and MCDA is identified as a practical decision-making tool in evaluating the BR balance of vaccines.
You can read the published manuscript here:
https://www.tandfonline.com/doi/full/10.1586/14760584.2016.1107480
Vulnerability bottleneck analysis (qualitative research)
Vulnerability bottleneck analysis (qualitative research)

This Project was commissioned by a steering committee to support the development of quality indicators on vulnerability. A mixed-methods approach was used, starting with a literature review and interviews with researchers, nurses and patient representatives, followed by the design and implementation of a questionnaire. A total of 170 responses were obtained and discussed in more detail in two focus groups. The project was completed with the development of a comprehensive report describing the results and advising on possible solutions and next steps.
Further details (in Dutch) here:
Conference reports (medical writing)
Conference reports (medical writing)

P95’s medical writers are frequently consulted to prepare meeting or conference reports for publication.
These are a few examples:
IABS/DCVMN webinar on next-generation sequencing
(https://www.sciencedirect.com/science/article/pii/S1045105622000811)
Virology, epidemiology, immunology and vaccine development of SARS-CoV-2, update after nine months of pandemic
(https://www.sciencedirect.com/science/article/pii/S1045105620301378?via%3Dihub)
Third human challenge trial conference, Oxford, United Kingdom, February 6–7, 2020, a meeting report
(https://www.sciencedirect.com/science/article/abs/pii/S1045105620300440?via%3Dihub)
Report of the second international conference on next-generation sequencing for adventitious virus detection in biologics for humans and animals
(https://www.sciencedirect.com/science/article/pii/S1045105620300634?via%3Dihub)
Human challenge trial workshop: Focus on quality requirements for challenge agents, Langen, Germany, October 22, 2019
(https://www.sciencedirect.com/science/article/pii/S1045105620300452?via%3Dihub)
HPV board meeting: Overcoming barriers in HPV vaccination and screening programs
(https://www.sciencedirect.com/science/article/pii/S2405852116300957?via%3Dihub)
Public health impact of low-dose aspirin on colorectal cancer, cardiovascular disease and safety in the UK (statistics/IT)
Public health impact of low-dose aspirin on colorectal cancer, cardiovascular disease and safety in the UK (statistics/IT)

An individual’s risk of cardiovascular disease and colorectal cancer may be decreased by prophylactic low-dose aspirin therapy. However, this therapy is also associated with adverse effects such as bleeding. To evaluate the expected population-level effect of low-dose aspirin prophylaxis, a micro-simulation model was built with data from UK governmental agencies and recent publications. The model demonstrated that the decrease in the incidence rate and the number of fatal cardiovascular disease and colorectal cancer events was larger than the increase in the incidence rate and number of fatal adverse effects in the UK population indicated for such prophylaxis. P95 contributed to the study design, data collection, model development, and study document writing and editing.
You can read the published manuscript here:
https://www.sciencedirect.com/science/article/pii/S2352906721001391?via%3Dihub
Effectiveness and safety of the Fiocruz ChAdOx COVID-19 vaccine used in a mass vaccination campaign in Botucatu, Brazil
Effectiveness and safety of the Fiocruz ChAdOx COVID-19 vaccine used in a mass vaccination campaign in Botucatu, Brazil
Mass vaccination can be an important tool to rapidly contain pathogens. In 2021, two doses of the recombinant COVID-19 vaccine ChadOx1-nCoV19 were offered to all 18–60-year-olds in Botucatu, Brazil, through a mass vaccination campaign. P95 contributed to a study that used this unique opportunity to assess the effectiveness and safety of the vaccine in real-world settings. The first and second vaccine doses were administered to 77,683 and 74,051 citizens, respectively. The effectiveness against COVID-19 disease of any severity was 74.5% after dose 1 and 81.3% after dose 2, which is comparable to the efficacy observed in clinical trials. Moreover, vaccination was well tolerated and did not raise safety concerns. Together, these results show the beneficial effect of this mass vaccination campaign in Botucatu.
Effectiveness and safety data were described in two publications:
https://www.ncbi.nlm.nih.gov/pubmed/36311567
https://www.sciencedirect.com/science/article/pii/S0264410X22010106?via%3Dihub
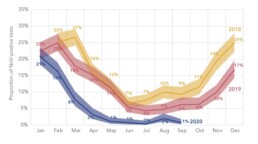
Decrease in norovirus infections in Germany following COVID-19 containment measures (epidemiology)
Decrease in norovirus infections in Germany following COVID-19 containment measures (epidemiology)
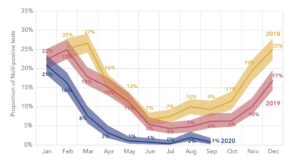
The preventive measures put in place to contain COVID-19 infections likely affected the spread of other pathogens, such as norovirus, a highly infectious virus that causes diarrheal disease. Between 2020-2021, P95 collaborated with a large laboratory in Germany to assess the effect of COVID-19 containment measures on the presence of norovirus. Data from routine testing for norovirus were analyzed and reported at P95. Compared to 2018–2019, the proportion of norovirus-positive stool samples was significantly lower in 2020, hovering around 0% from May to December. This likely reflects the impact of COVID-19 containment measures adopted since March 2020.
You can read the published manuscript here:
https://www.ncbi.nlm.nih.gov/pubmed/33581238
Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in Europe and Latin America
Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in Europe and Latin America
The mRNA vaccines against SARS-CoV-2 have protected billions of people worldwide, but the novel variants of concern (VoC) could escape the vaccine-acquired immunity. P95 participated in the safety monitoring of the phase 2b/3 HERALD study to weigh the efficacy and safety of the unmodified mRNA COVID-19 vaccine candidate, CVnCoV. In this 40,000-person trial across ten countries in Europe and Latin America, CVnCoV was found to be 48% efficacious against symptomatic COVID-19. Systemic adverse events were more frequent in the CVnCoV group than in the placebo group. Judging the fast-changing COVID-19 vaccine landscape with the emergence of VoC, the researchers recommended halting activities on CVnCoV and aiming to develop next-generation vaccine candidates.
You can read the published manuscript here:
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00677-0/fulltext
Economic burden of varicella in Europe
Economic burden of varicella in Europe
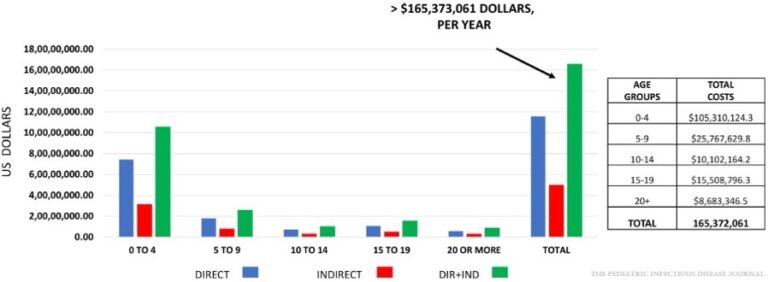
Varicella is a highly contagious infection that yearly isolates millions of children at home for over a week. This causes a high morbidity and economic burden, which can greatly be prevented with vaccination. P95 collaborated on a systematic review that first estimated the number of cases of varicella across Europe and the cost of varicella at more than 600 million euros annually across 31 European countries in the absence of universal vaccination.
Full results can be accessed in the following publications:
https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2445-2https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-021-12343-x
Lyme disease systematic reviews
Lyme disease systematic reviews

Lyme disease, a tick-borne infection, has been increasing in incidence and expanding to new regions, partly due to climate change. Since 2020, P95 has been collaborating on a major systematic literature review of studies on Lyme disease. Publications and grey literature sources have been searched for data on Lyme incidence, epidemiology, hospitalization and clinical manifestations, among other outcomes. Data are being compiled for different regions of the World. This major study is expected to result in several publications and congress communications.
A first publication is already available, and three others are expected to be published soon:
https://www.sciencedirect.com/science/article/abs/pii/S1877959X22001418